Powering the Future: Unlocking the Potential of Hydrogen Fuel Cells
“Energy runs the world. You have to have secure and reliable sources of energy to move all of the modern processes, whether it be our laptops or our cars.” – Patrick Ward, SRNL Scientist

Patrick Ward has a $3 million grant to research new methods of hydrogen storage.
photo: Savannah River Site Photography
The United States is working toward a power sector free of carbon pollution by 2035. Achieving this ambitious goal requires a commitment to developing renewable energy sources that are commercially competitive.
Savannah River National Laboratory (SRNL) Scientist Patrick Ward is supporting the drive toward a more secure energy future and less carbon pollution by working to make hydrogen fuel cells a more viable option for the transportation sector. In 2022, Ward received a $3 million Department of Energy (DOE) Office of Science Basic Energy Science Program award to further fundamental research aimed at enabling new pathways for hydrogen storage and production technologies.
Hydrogen is particularly attractive because it is a cleaner alternative to greenhouse gas-producing fossil fuels and not tied to weather conditions like solar or wind power.
“Hydrogen has a lot of potential because we could convert water into hydrogen and oxygen, use it to propel our vehicles, and then turn it right back into water,” says Ward.
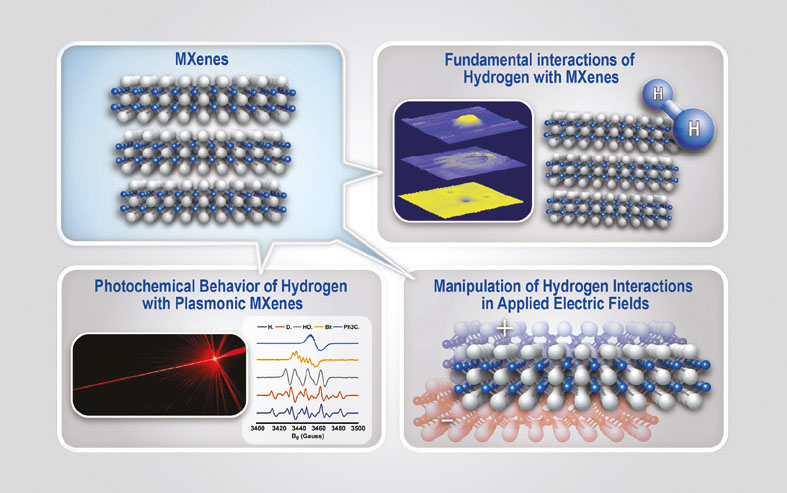
However, it has been challenging to find ways to store and deploy hydrogen safely and efficiently. Ward’s research is exploring how to best store hydrogen molecules within the spaces of MXenes, a two-dimensional transition metal carbide, carbonitride, or nitride material. Storing the hydrogen in solid-state materials would be a safer alternative to storing as a compressed gas.
“We’re looking at the fundamental interactions between MXenes and hydrogen and trying to understand how we can fine tune these interactions to find the sweet spot in binding hydrogen to the surface. This would allow us to store hydrogen under ambient conditions,” says Ward.
graphic: Savannah River National Laboratory
“Now it’s a too hot or too cold problem. Metal organic frameworks are highly porous materials with high surface areas, but you have to cool your tank down to cryogenic temperatures with liquid nitrogen, or you have these materials that require very high temperatures to pull the hydrogen off. We’re basically trying to map out and understand how we tune materials to bind in that middle temperature range.”
Ward currently is investigating how hydrogen diffuses into MXenes and the role defects in the material play in the diffusion pathway.
“One of the challenges with hydrogen storage is that you must be able to go to the pump and fill up your car with hydrogen in a reasonable amount of time. If you have to sit there for five or six hours, it’s not really feasible anymore. It’s not competitive with a gas vehicle,” says Ward. “Understanding diffusion in these materials allows us to understand how you can rapidly store hydrogen in them. Having more information about how hydrogen uptakes into materials will give us the insight to tune for the ideal parameters.”
The project will also explore how light, and the excitation of surface plasma resonances influence hydrogen dissociation of the surface and how electric fields play a role in the interaction strength between hydrogen and the material.
According to Ward, SRNL is particularly well-suited for this work because of its unique instrumental capabilities.
“We have a large amount of equipment related to being able to measure hydrogen uptake in materials, kinetics and decomposition temperatures, as well as specialized equipment located in argon-filled glove boxes for air sensitive materials,” says Ward.
To address these scientific challenges, an excellent research team was assembled between SRNL and University collaborators: Yury Gogotsi from Drexel University, Kah Chun Lau from California State University Northridge and Paul Weiss from University of California, Los Angeles.

SRNL’s Hydrogen Technology Research Laboratory possesses specialized equipment, such as several custom hydrogen sorption systems.
photo: SRNL Communications
Ward says the biggest challenge facing hydrogen power is commercial infrastructure.
“It’s sort of a chicken or the egg problem. No one wants to invest in the fueling stations if there are no cars, and no one wants to invest in the cars if there are no fueling stations,” says Ward.
“Hydrogen fuel cell vehicles in the United States are limited by the availability of hydrogen fueling stations and large-scale hydrogen production. It is well-known that a fueling infrastructure and abundant hydrogen availability is required to encourage mass production and initiate the deployment of fuel cell vehicles.”
In answer to this challenge, DOE’s H2Hubs program aims to establish six regional green hydrogen production hubs in the U.S., providing the critical infrastructure needed to encourage hydrogen fueling station investments and fuel cell vehicle production.
“Energy runs the world. You have to have secure and reliable sources of energy to move all of the modern processes, whether it be our laptops or our cars. By finding alternative sources of energy, we’re supporting the endeavor of ensuring energy security for the nation,” says Ward.

Thermogravimetric analysis equipment in inert environments.
photo: SRNL Communications