Tech Briefs
Savannah River National Laboratory
Sulfur Accumulation Prevention In Membrane Electrode Assembly
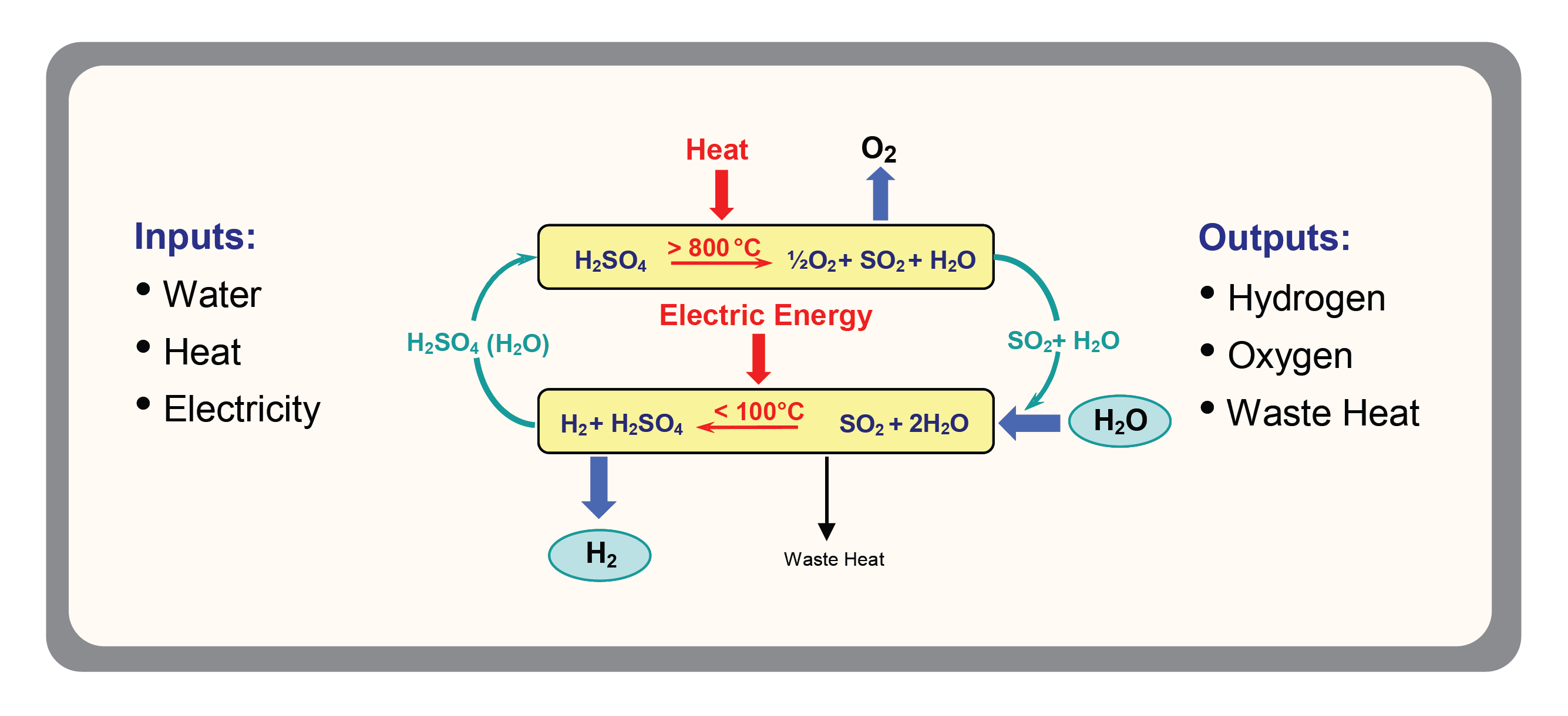
Hydrogen can be produced in a hybrid sulfur cycle that utilizes an electrolyzer cell that has both an anode and a cathode. The hybrid sulfur cycle is a hybrid thermochemical cycle that can be used in conjunction with advanced nuclear reactors or centralized solar receivers to produce hydrogen by water splitting. The chemistry involves only sulfur compounds, water, hydrogen and oxygen.
Using sulfur dioxide, water can be electrolyzed to produce hydrogen at a significantly lower voltage than is required for conventional water electrolysis. The byproduct of the reaction – sufuric acid – can then be decomposed at high temperature to produce oxygen and regenerate sulfur dioxide. The use of an electrolyzer cell requires a greater amount of voltage over time to drive the reaction.
Over time, the process could become less efficient due to the formation of a sulfur layer between the cathode and the membrane. The presence of the sulfur layer adds ohmic resistance to the membrane electrode assembly, which increases cell voltage, and presses the membrane electrode assembly into anode flow passages that increases pressure drop. Aside from increasing the voltage necessary to drive the production of hydrogen, the presence of the sulfur layer acts to delaminate the membrane electrode assembly and reduce the life of the apparatus.
Hydrogen may be potentially used to replace gasoline as a transportation fuel, possibly generating a need for millions of tons of hydrogen per year. Therefore it is important to eliminate or reduce the sulfur layer accumulation in this process makes it possible for the commercial implementation of efficient hydrogen production using the hybrid sulfur cycle.
Background
This invention provides a method to conceal identification symbols (i.e. symbols, bar codes, numbers, letters, etc.) within an object and retrieval of information by X-ray inspection without destructively dismantling or testing the object. The internal placement makes it difficult to remove or alter identification information. These properties would be useful in protecting valuable property by deterring theft, identifying lost or stolen property, or tagging and tracking of property and sealed containers during shipment.
At a glance
- Prolongs equipment life
- Lowers the production voltage and cost
- U. S. Patent 8,709,229
How it works
Sulfur dioxide and deionized water may be used as anode reactants to form sulfuric acid. The electrolyzer cell includes a membrane that allows the hydrogen ions produced at the anode to pass through while preventing hydrogen gas, sulfuric acid or other chemicals from passing through. The hydrogen gas generated at the cathode can be collected. Water is introduced at the cathode to maintain hydration, allowing generation of hydrogen at a much lower voltage than conventional electrolysis. Therefore much less energy is required for hydrogen production resulting in significantly less cost.
Partnering opportunities
SRNL invites interested companies with proven capabilities in this area of expertise to develop commercial applications for this process under a cooperative research and development agreement (CRADA) or licensing agreement. Interested companies will be requested to submit a business plan setting forth company qualifications, strategies, activities, and milestones for commercializing this invention. Qualifications should include past experience at bringing similar products to market, reasonable schedule for product launch, sufficient manufacturing capacity, established distribution networks, and evidence of sufficient financial resources for product development and launch.
Download Tech Brief
Contact Information
Savannah River National Laboratory
E-mail: partnerships@srnl.doe.gov