Tech Briefs
Savannah River National Laboratory
Controlled Equilibrium Catalytic Hydrogen Isotope Exchange (CECIE)
Technology Overview
Savannah River National Laboratory has developed a continuous approach to catalytic hydrogen isotope exchange where the catalytic reactor is connected to hydrogen isotope processing equipment to continuously recirculate hydrogen isotopes back to the reactor. CECIE is applicable to a diverse array of industries. For example, replacing the normal protium (H) in an organic molecule with deuterium (D) or tritium (T) creates “tracers” to better understand chemical, biological, and pharmaceutical systems. Deuterated organics have also found use in organic light-emitting diodes (OLEDs) and optical fibers as means to increase stability and reduce data transmission losses, respectively. For fusion energy, deuterated polymers are critical for inertial fusion targets, and CECIE may also be used for catalytic removal of tritium from process oils and lubricants.
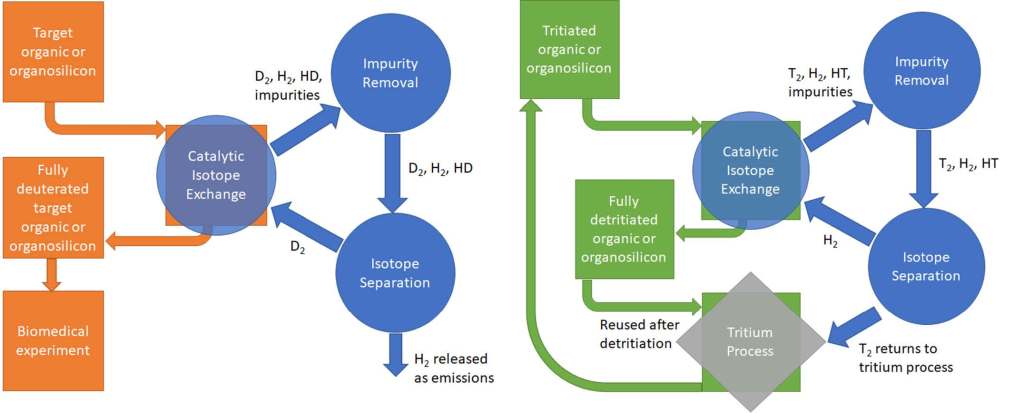
Figure 1. (left) Representative flow diagram for the deuteration of an organic or an organosilicon molecule to be used in a biomedical experiment. The orange blocks and arrows represent the organic/organosilicon flow, while the blue blocks show the D2 flow, with recovery. (right) Representative flow diagram for the detritiation of an organic or an organosilicon molecule that was exposed to tritium while serving as a tritium processing component. The green blocks and arrows represent the organic/organosilicon flow, while the blue blocks show the T2 flow, with recovery.
Benefits
- Faster and more complete hydrogen isotope exchange
- Minimizes losses of valuable hydrogen isotopes
- Integrates with existing infrastructure in fusion facilities
Applications and Industries
- Relevant industries: fusion, fission, defense, pharmaceutical, biomedical, LED, semiconductor, optical fiber, polymer.
Description
Historically, the production of isotopically exchanged organic material through catalytic isotope exchange is done by exposing an organic molecule-catalyst mixture in a heated, sealed vessel to D2 gas or D2O liquid. This batch process is inefficient and insufficient for the scalable production of highly deuterated organics as hydrogen isotope exchange is governed by the fundamental equilibria between the isotopologues in the two phases. Another issue is that the high pressure, high temperature environment of the sealed vessel may change the molecular structure of the target molecule. Alternatively, some deuterated organic molecules may be synthesized from deuterated precursors. However, the types of molecules that can be achieved in this manner are constrained by known chemical reaction pathways and the availability of the necessary precursors. Further, this approach is not applicable to detritiation of organics with entrained tritium. Thus, there remains a need to achieve selectively or highly exchanged complex organic molecules in a manner that does not require altering chemical structures.
A continuously purged system is one way to circumvent the equilibrium limitation of isotope exchange reactions. In this case, a pure hydrogen isotope stream (e.g., D2) continuously flows into the reactor and interfaces with the organic or organosilicon molecules and catalysts. The outlet flow carries away both the exchanged and unexchanged hydrogen isotopes, which continuously keeps the equilibrium favorable for isotope exchange. It is important to note that sealed, batch systems conserve deuterium and tritium, while a traditional flow-through system would “waste” a significant amount of these precious resources. Further, in the case of the tritiation and detritiation, the outlet flow would be an environmental health hazard due to the presence of tritium. However, if the flow-through system is coupled with a hydrogen isotope processing system, as in the CECIE process, then the outlet flow can be readily processed to recover the deuterium or tritium, negating these issues. For the detritiation of organic and organosilicons in tritium processing facilities, the catalytic isotope exchange process can be coupled with the existing process infrastructure to manage the tritium effluent. For catalytic deuteration/tritiation, a flexible hydrogen isotope processing system can be coupled with the reactor.
Intellectual Property
- Patent U.S. 11,981,613B2
- Development Stage: Under development
- Available for licensing and CRADAs
Partnering opportunities
SRNL invites interested companies with proven capabilities in this area of expertise to develop commercial applications for this process under a cooperative research and development agreement (CRADA) or licensing agreement. Interested companies will be requested to submit a business plan setting forth company qualifications, strategies, activities, and milestones for commercializing this invention. Qualifications should include past experience at bringing similar products to market, reasonable schedule for product launch, sufficient manufacturing capacity, established distribution networks, and evidence of sufficient financial resources for product development and launch.
Download Tech Brief
Contact Information
Savannah River National Laboratory
E-mail: SRNL-Partnerships@srnl.doe.gov